
Advancements in Ocular Hypertension Clinical Trial Pipeline as 60+ Companies Pave the Way for Future Solutions | DelveInsight
The ocular hypertension market is growing due to the rising prevalence of conditions impairing aqueous humor outflow, such as scarring and inflammation, leading to elevated intraocular pressure (IOP). Advancements in treatment, including sustained-release implants and micro-dosing technologies, are improving patient compliance and outcomes. Enhanced imaging techniques like optical coherence tomography (OCT) support early diagnosis and timely intervention, further driving market growth. This combination of factors underscores strong potential for continued expansion in this segment.
/EIN News/ -- New York, USA, Jan. 20, 2025 (GLOBE NEWSWIRE) -- Advancements in Ocular Hypertension Clinical Trial Pipeline as 60+ Companies Pave the Way for Future Solutions | DelveInsight
The ocular hypertension market is growing due to the rising prevalence of conditions impairing aqueous humor outflow, such as scarring and inflammation, leading to elevated intraocular pressure (IOP). Advancements in treatment, including sustained-release implants and micro-dosing technologies, are improving patient compliance and outcomes. Enhanced imaging techniques like optical coherence tomography (OCT) support early diagnosis and timely intervention, further driving market growth. This combination of factors underscores strong potential for continued expansion in this segment.
DelveInsight’s 'Ocular Hypertension Pipeline Insight 2025' report provides comprehensive global coverage of pipeline ocular hypertension therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the ocular hypertension pipeline domain.
Key Takeaways from the Ocular Hypertension Pipeline Report
- DelveInsight’s ocular hypertension pipeline report depicts a robust space with 60+ active players working to develop 70+ pipeline ocular hypertension drugs.
- Key ocular hypertension companies such as Nicox, Qlaris Bio, TheratOcular Biotek Co., Ltd., Ocular Therapeutix, JeniVision, Inc., Santen, VivaVision Biotech, ONL Therapeutics, MediPrint Ophthalmics, Sun Pharma Advanced Research Company, Ripple Therapeutics, Peregrine Ophthalmic, Chong Kun Dang, Laboratoires Thea, Novoron Bioscience, Alcon, and others are evaluating new ocular hypertension drugs to improve the treatment landscape.
- Promising pipeline ocular hypertension therapies such as NCX-470, PDP716, QLS-101, TO-O-1001, OTX-TIC, AGN-193408, LL-BMT1, DE-126, ONL1204, DE-130A, VVN539, POLAT-001, RTC-1119, D565, Bimatoprost, NOVO-118, AR-17043, and others are under different phases of ocular hypertension clinical trials.
- In December 2024, Nicox announced that its Denali Phase III trial, evaluating the efficacy and safety of NCX470 in patients with open-angle glaucoma or ocular hypertension, is now fully enrolled in China and screening has been closed.
- In November 2024, MediPrint Ophthalmics announced the results of the Company’s Phase IIb clinical trial of LL-BMT1. Employing a novel 3D printed, drug-eluting contact lens for sustained delivery of bimatoprost and hyaluronic acid, the clinical trial achieved all Phase IIb endpoints.
- In October 2023, SpyGlass Pharma announced the initiation of a Phase I/II clinical trial investigating its intraocular drug delivery platform in patients with glaucoma and visually significant cataracts. The SpyGlass platform is designed to be implanted at the time of routine cataract surgery and deliver multiple years of bimatoprost, to lower intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
- In April 2024, Qlaris Bio Completed 24 Million USD Series B Financing Round to Advance QLS‑111, a First-in-class IOP-lowering Drug Candidate for Glaucoma.
- In April 2024, Qlaris Bio announced the initiation and dosing of two separate US Phase II masked, randomized clinical trials investigating QLS‑111 in patients with ocular hypertension and glaucoma.
- In March 2024, Nicox announced results from the Mont Blanc pivotal Phase IIItrial comparing NCX 470 to latanoprost in the lowering of intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension have been published in the peer-reviewed journal American Journal of Ophthalmology.
Request a sample and discover the recent advances in ocular hypertension drugs @ Ocular Hypertension Pipeline Report
The ocular hypertension pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage ocular hypertension drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the ocular hypertension clinical trial landscape.
Ocular Hypertension Overview
Ocular hypertension occurs when the pressure inside the eye exceeds the normal range, surpassing 21 mm Hg. This rise in intraocular pressure, which tends to increase with age but can also result from other conditions, is a significant risk factor for glaucoma. Consequently, individuals with ocular hypertension are more likely to develop glaucoma. However, the two conditions are distinct: ocular hypertension refers to elevated intraocular pressure without damage to the optic nerve, whereas glaucoma involves optic nerve damage, which can occur even when intraocular pressure is normal or elevated. Glaucoma can lead to visual field loss and, in advanced stages, even central vision impairment.
Ocular hypertension arises from a dysfunction in the drainage system for aqueous humor—the fluid responsible for nourishing and maintaining ocular structures. When this fluid does not drain properly, the balance between its production and outflow is disrupted, resulting in increased intraocular pressure. This rise is typically gradual but can sometimes occur suddenly.
To diagnose ocular hypertension, the following tests are recommended:
- Central visual field evaluation using standard automated perimetry.
- Optic nerve and fundus examination with stereoscopic slit lamp biomicroscopy (with pupil dilation if needed).
- Optical coherence tomography (OCT) or optic nerve head imaging.
- Intraocular pressure (IOP) measurement using Goldmann applanation tonometry.
- Assessment of peripheral anterior chamber configuration and depth through gonioscopy.
The primary treatment for ocular hypertension involves eye drops, similar to those used for glaucoma. These medications lower intraocular pressure by either reducing the production of aqueous humour by the ciliary body or enhancing its drainage. Recent advancements have made these treatments more effective and less prone to side effects. Common medications include prostaglandins, beta-blockers, alpha-adrenergic agonists, carbonic anhydrase inhibitors, rho kinase inhibitors, and miotic or cholinergic agents. If eye drops fail to reduce IOP adequately, other measures such as laser or surgical interventions may be necessary.
Ultimately, patients with ocular hypertension require long-term monitoring to assess the potential progression to glaucoma and to evaluate the effectiveness of their treatment plan. Regular follow-ups with a physician are essential to ensure optimal eye health and prevent complications.
Find out more about ocular hypertension drugs @ Ocular Hypertension Analysis
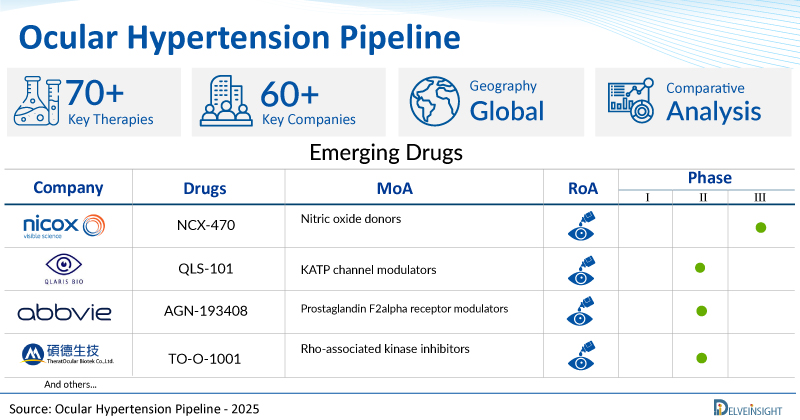
A snapshot of the Pipeline Ocular Hypertension Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| NCX-470 | Nicox | III | Nitric oxide donors | Ophthalmic |
| QLS-101 | Qlaris Bio | II | KATP channel modulators | Ophthalmic |
| AGN-193408 | AbbVie | II | Prostaglandin F2alpha receptor modulators | Ophthalmic |
| TO-O-1001 | Theratocular Biotek | I/II | Rho-associated kinase inhibitors | Ophthalmic |
| ONL1204 | ONL Therapeutics | I | Apoprotein inhibitors; CD95 antigen inhibitors; Fas ligand protein inhibitors | Ophthalmic |
Learn more about the emerging ocular hypertension therapies @ Ocular Hypertension Clinical Trials
Ocular Hypertension Therapeutics Assessment
The ocular hypertension pipeline report proffers an integral view of the emerging ocular hypertension therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Ocular Hypertension Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intra-articular, Intraocular, Intrathecal, Intravenous, Ophthalmic, Oral, Parenteral, Subcutaneous, Topical, Transdermal
- Therapeutics Assessment By Molecule Type: Oligonucleotide, Peptide, Small molecule
- Therapeutics Assessment By Mechanism of Action: Nitric oxide donors, KATP channel modulators, Rho-associated kinase inhibitors, Alpha 2 adrenergic receptor agonists, Beta-adrenergic receptor antagonists, Prostaglandin F2alpha receptor modulators
- Key Ocular Hypertension Companies: Nicox, Qlaris Bio, TheratOcular Biotek Co., Ltd., Ocular Therapeutix, JeniVision, Inc., Santen, VivaVision Biotech, ONL Therapeutics, MediPrint Ophthalmics, Sun Pharma Advanced Research Company, Ripple Therapeutics, Peregrine Ophthalmic, Chong Kun Dang, Laboratoires Thea, Novoron Bioscience, Alcon, and others.
- Key Ocular Hypertension Pipeline Therapies: NCX-470, PDP716, QLS-101, TO-O-1001, OTX-TIC, AGN-193408, LL-BMT1, DE-126, ONL1204, DE-130A, VVN539, POLAT-001, RTC-1119, D565, Bimatoprost, NOVO-118, AR-17043, and others.
Dive deep into rich insights for new ocular hypertension treatments, visit @ Ocular Hypertension Drugs
Table of Contents
| 1. | Ocular Hypertension Pipeline Report Introduction |
| 2. | Ocular Hypertension Pipeline Report Executive Summary |
| 3. | Ocular Hypertension Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Ocular Hypertension Clinical Trial Therapeutics |
| 6. | Ocular Hypertension Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Ocular Hypertension Pipeline: Late-Stage Products (Phase III) |
| 8. | Ocular Hypertension Pipeline: Mid-Stage Products (Phase II) |
| 9. | Ocular Hypertension Pipeline: Early-Stage Products (Phase I) |
| 10. | Ocular Hypertension Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Ocular Hypertension Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Ocular Hypertension Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the ocular hypertension pipeline therapeutics, reach out @ Ocular Hypertension Therapeutics
Related Reports
Ocular Hypertension Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key ocular hypertension companies, including JeniVision, Inc., Nicox Ophthalmics, Inc., Laboratoires Thea, Glaukos Corporation, D. Western Therapeutics Institute, Inc., AbbVie, TearClear Corp, Ocular Therapeutix, Inc., Perfuse Therapeutics, Inc., among others.
Ocular Hypertension Epidemiology Forecast
Ocular Hypertension Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted ocular hypertension epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Hypertension Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key hypertension companies, including Quantum Genomics, CinCor Pharma, Mineralys Therapeutics, Alnylam Therapeutics, Ionis Pharmaceuticals, Future Medicine, Pharmosa BioPharm, Aerovate Therapeutics, Novartis, Cereno Scientific AB, Torrent Pharmaceuticals Limited, JeniVision, Inc., Merck Sharp & Dohme LLC, AbbVie, Acceleron Pharma Inc., Hanmi Pharmaceutical Company Limited, Gossamer Bio Inc., Shanghai Pharmaceuticals Holding Co., Ltd, Insmed Incorporated, Gmax Biopharm LLC., Altavant Sciences GmbH, Bayer, Respira Therapeutics, Inc., Aadi Bioscience, Inc., Boehringer Ingelheim, JW Pharmaceutical, PRM Pharma, LLC, PolyActiva Pty Ltd, pH Pharma, Nicox Ophthalmics, Inc., Ocular Therapeutix, Inc., Santen SAS, Whitecap Biosciences, LLC, Chong Kun Dang Pharmaceutical, AJU Pharm Co., Ltd., Laboratoires Thea, Aerami Therapeutics, KBP Biosciences, Cumberland Pharmaceuticals, Vigonvita Life Sciences, IlDong Pharmaceutical Co Ltd, Qlaris Bio, Inc., Santen Pharmaceutical, VivaVision Biotech, Mitsubishi Chemical Group Corporation., Idorsia Pharmaceuticals, United Therapeutics Corporation, VIVUS LLC., PhaseBio Pharmaceuticals, Vascular Biosciences, CAR peptide, Liquidia Corporation, Centessa Pharmaceuticals, Claritas Pharmaceuticals, Halo Biosciences, PulmoSIM Therapeutics, Keros Therapeutics, among others.
Pulmonary Hypertension Pipeline
Pulmonary Hypertension Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key pulmonary arterial hypertension companies, including Reata Pharmaceuticals, Pharmosa BioPharm, United Therapeutics Corporation, Acceleron Pharma, Bellerophon Therapeutics, Dong-A ST, Mezzion, Northern Therapeutics, Merck Sharp & Dohme Corp., Apeiron Biologics, VIVUS, Respira Therapeutics, Resverlogix, Bial - Portela C S.A., Vigonvita Life Sciences, Altavant Sciences, Roche, PhaseBio Pharmaceuticals, Gossamer Bio, Reviva Biopharmaceuticals, Cereno Scientific, Attgeno, Rho Federal Systems Division, Inc., Cumberland Pharmaceuticals, AstraZeneca, Aerovate Therapeutics, Celon Pharma, Camurus, ATXA Therapeutics, Gmax Biopharma, Aerami Therapeutics, Pfizer, Aadi Biosciences, SystImmune, Claritas Pharmaceuticals, Reviva Biopharmaceuticals, Aqualung Therapeutics, Pulmokine, Recursion Pharmaceuticals, among others.
Pulmonary Arterial Hypertension Pipeline
Pulmonary Arterial Hypertension Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key pulmonary arterial hypertension companies, including Merck Sharp & Dohme, Acceleron Pharma, Liquidia Technologies, Gossamer Bio, Resverlogix, PhaseBio Pharmaceuticals, Pharmosa BioPharm, Complexa, Gmax Biopharm Australia, Mezzion, Radikal Therapeutics, Galectin Therapeutics, Altavant Sciences, Ribomic, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

